Product Special
Refresh your eyes with Upneeq, now only $159!
*Add to cart and discount will apply.

If you have Glaucoma or any other eye disease, please check with your ophthalmologist before using Upneeq®.
Upneeq® has not been tested on pregnant women.
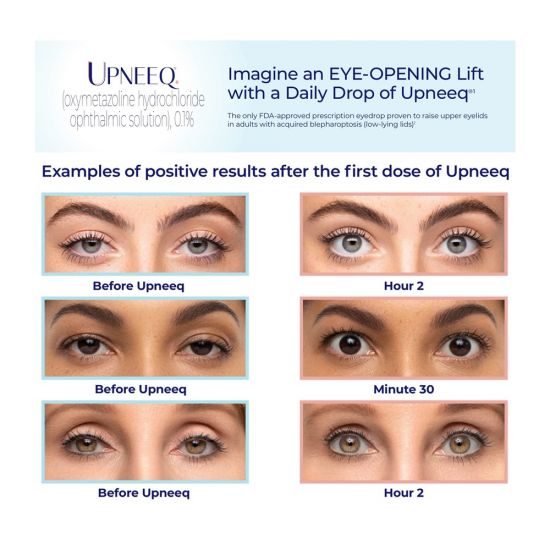
Upneeq® is the only FDA-approved prescription eye drop that lifts your upper eyelids to open your eyes. Dr. Klein and OC Dermatology were selected to be the first dermatology office in Orange County, and one of the first in the U.S., to offer this game-changer for droopy eyelids.
| Benefits |
|
|---|---|
| FAQs | What warnings and precautions are associated with UPNEEQ®?
Do not let the tip of the UPNEEQ® vial touch your eye or any other surface. This can help prevent eye injury or contamination. Each UPNEEQ® vial is for one-time use and should be discarded after being used. What are the most common side effects of UPNEEQ®? The most common adverse reactions with UPNEEQ® (occurring in 1-5% of patients) were eye inflammation, eye redness, dry eye, blurred vision, eye pain at the time of use, eye irritation, and headache. What should my doctor know about before prescribing me UPNEEQ®? Your doctor should review your full medical history before prescribing UPNEEQ®. Patients who use a certain class of antidepressant medication (monoamine oxidase inhibitors) should also be careful when using UPNEEQ®, as it may affect the way your body absorbs the medication. These are not all of the possible side effects of UPNEEQ®. Tell your doctor if you have any side effect that bothers you or does not go away. Call your doctor for medical advice about side effects. |
| Skin Type | Body Areas |
| Size | 0.3 mL |
| Brand | Private Label |
Apply one drop of Upneeq in each affected eye as directed, once a day.
Each mL of Upneeq® contains 1 mg of oxymetazoline hydrochloride, equivalent to 0.9 mg (0.09%) of oxymetazoline free base.